Ultrafiltration Ultrafiltration is usually a technologies most frequently employed in pharmaceutical water methods for eliminating endotoxins from the water stream. It may also use semipermeable membranes, but in contrast to RO, these typically use polysulfone membranes whose intersegmental “pores” have been purposefully exaggerated through their manufacture by protecting against the polymer molecules from achieving their more compact equilibrium proximities to one another.
It carries a considerably less stringent specification for bacterial endotoxins than Sterile Water for Injection, and thus, isn't ideal for parenteral programs. Nonmonographed Producing Waters In addition to the bulk monographed waters explained over, nonmonographed waters can be used in pharmaceutical processing measures for instance cleansing, artificial methods, or even a starting off materials for even more purification. The subsequent is an outline of various of these nonmonographed waters as cited in numerous destinations within this compendia.
Deaerated Water is used in both dissolution tests as well as liquid chromatography apps where by outgassing could both interfere Together with the Evaluation by itself or cause erroneous success as a consequence of inaccurate volumetric withdrawals.
, vacuum filtering via a 0.forty five-µm rated membrane, and vigorously stirring the filtrate whilst maintaining the vacuum. This chapter exclusively suggests that other validated techniques may be used. In other monographs that also tend not to point out Deaerated Water by identify, degassing of water and various reagents is achieved by sparging with helium. Deaerated Water is used in equally dissolution tests along with liquid chromatography programs the place outgassing could possibly interfere Along with the Examination by itself or lead to faulty outcomes on account of inaccurate volumetric withdrawals. Programs the place ambient temperature water is used for reagent planning, nevertheless the checks are executed at elevated temperatures, are candidates for outgassing consequences. If outgassing could interfere with examination overall performance, like chromatographic movement, colorimetric or photometric measurements, or volumetric precision, then Deaerated Water ought to almost certainly be used, no matter whether known as for in the Assessment or not.
Microbial contamination of oral liquid and topical drug products and solutions carries on to generally be a significant issue, and will likely be rooted in the use of contaminated water.
For apparent good reasons, the biopharmaceutical industry is The most intently controlled, and every ingredient of the pharmaceutical generation system must be very carefully purified and monitored to circumvent the contamination of products. From investigate and enhancement into the manufacturing of biopharmaceutical products, biopharmaceutical water purification programs Perform a basic role in each and every phase of biopharmaceutical operations.
as creating no drastically interfering gasoline chromatography peaks. Referenced monographs specify working with this water because the solvent with the planning of normal and check alternatives with the Residual solvents test. Direct-Cost-free Water— This water is used for a transferring diluent for an analyte in a very Lead
Pharmaguideline is really a pharmaceutical web site where pharmaceutical concepts are defined in very simple and simply understandable language for experts and students. All posts and SOPs are created by Ankur Choudhary.
Filtering the blood in hemofiltration: WFI is likewise used within the removing of waste solutions within the blood and the injection of sterile replacement fluids.
These programs have to have frequent sanitization and microbiological monitoring to be sure water of correct microbiological good quality within the points of use. The Purified Water monograph also lets bulk packaging for professional use somewhere else. When this is done, the essential specifications are All those from the packaged water Sterile Purified Water, aside from Sterility and Labeling. There is a potential for microbial contamination and also other excellent modifications of the bulk packaged get more info nonsterile water to come about. As a result, this form of Purified Water should be geared up and saved in this type of fashion that limits microbial growth and/or just used in a timely trend right before microbial proliferation renders it unsuitable for its meant use. Also depending on the materials used for packaging, there may very well be extractable compounds leaching into the water within the packaging. However this short article may well fulfill its essential chemical attributes, this kind of extractables may possibly render the water an inappropriate option for some applications. It is the consumer's responsibilitiy to guarantee Health to be used of this packaged write-up when used in production, medical, or analytical apps the place the pure bulk kind of the water is indicated.
Bulk Monographed Waters and Steam The subsequent waters are typically manufactured in massive quantity by a numerous-unit Procedure water system and dispersed by a piping program to be used at exactly the same website.
Microbial specifications are generally assessed by take a look at strategies that get at the least forty eight to seventy two several hours to generate final results. Due to the fact pharmaceutical waters are frequently made by constant processes and used in merchandise and producing procedures quickly after generation, the water is likely to are already used perfectly prior to definitive examination effects can be obtained. Failure to meet a compendial specification would call for investigating the impact and generating a pass/fail determination on all solution lots amongst the past sampling's suitable test result in addition to a subsequent sampling's appropriate check consequence. The technological and logistical issues made by a hold off in the result of these kinds of an Investigation usually do not eliminate the consumer's have to have for microbial technical specs. Therefore, these water methods must be operated and taken care of inside of a controlled fashion that requires the method be validated to offer assurance of operational stability Which its microbial characteristics be quantitatively monitored in opposition to set up notify and motion levels that would provide an early sign of technique Regulate. The issues of water method validation and alert/action ranges and specs are included in this chapter.
. These procedures entail uncomplicated sparging of your liquid with an inert gasoline for example nitrogen or helium followed by inert gasoline blanketing to prevent oxygen reabsorption. The sparging periods cited vary from five to 15 minutes to an unspecified period of time. Some Purified Water and Water for Injection systems deliver water which is managed in a very hot condition and that's inert fuel blanketed in the course of its preparing and storage and distribution.
The results confirmed how on Restoration, it had been achievable to visualise compacted aggregates depending upon the Preliminary mobile density. By growing enough time (forty eight h), it may be observed how unique cells might be noticed. Interestingly, these specific cells confirmed elongated shapes, particularly in the situation of migrated cells from SHS fashioned at the very best cell density. By expanding time, cells homogeneously distributed throughout the floor. The time necessary to form a monolayer relies on initial cell density on SHS-derived 3D check here aggregates.
 Jonathan Taylor Thomas Then & Now!
Jonathan Taylor Thomas Then & Now! Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now!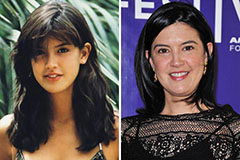 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Jaclyn Smith Then & Now!
Jaclyn Smith Then & Now!